Alloy Definition
Alloy: verb, past tense: alloyed; past participle: alloyed
mix (metals) to make an alloy.
“alloying tin with copper makes bronze”
What is a Metal Alloy?
An alloy is a mixture of two or more elements with at least one metal that lends its properties to the total compound. An alloy occurs when the properties that happen with the addition cause a change in a property that is beneficial. It is possible to mix two metallic substances and form a mixture of metals that is not an alloy. A metal alloy retains all the metal properties in the resulting combined material; electrical conductivity, tensile strength, ductility, opacity, and luster.

How is an alloy different from a pure metal?
Metal alloys are classified into two types: substitutional alloys, in which one metal replaces another and interstitial alloys, in which one metal fits between the spaces in another metal
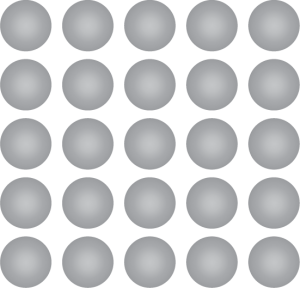
Pure Metal
Pure metal elements are metals that have not been alloyed with other metallic elements.
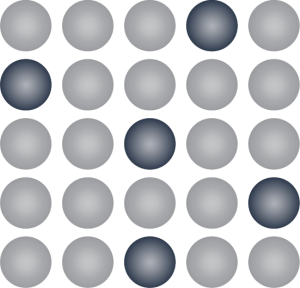
Substitutional Alloys Component atoms are the same size. One metal takes the place of another.
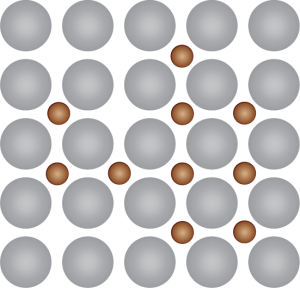
Interstitial Alloys Component atoms are different sizes. One metal fits in-between the spaces in another metal,
Alloys vs Pure Metal
There are many advantages to using alloys vs. pure metals. Pure metal elements are metals that have not been alloyed with other metallic elements. Many pure metals are soft. An alloy tends to be harder/stronger and more durable than pure metal because the mixture has atoms of different sizes. Alloys also provide high strength, resist corrosive environments, and improve appearance, as they are lustrous and contain a better finish.
Common Alloys and their Applications
Most of us encounter alloys in our daily life. Alloys are present in hardware, cars, gears, pistons, silverware, cookware, jewelry, tools and more. We will help you select the best alloy for your product. Below are a few examples of alloys we pour.
A242 Aluminum
Typical applications
535 Aluminum/Magnesium
C905 Tin/Bronze
Typical applications
C955 Nickel/Aluminum/Bronze
Typical applications
C863 Manganese/Bronze
Typical applications
Metal Alloy FAQ
An alloy is a mix or solution of at least one metal element and another metal or other element. Alloys include materials such as brass, pewter, bronze, white gold, rose gold and steel.
Copper-based alloys have the metal copper as their principal component. They’re highly resistance to corrosion. The best known copper alloys are bronze and brass. A bronze alloy is copper plus tin. A brass alloy is copper plus zinc. Copper is also added to precious metals like gold and silver resulting in alloys like rose, red, pink and white gold.
Alloys blend the best of the metals and elements they incorporate. Also, alloys can exhibit unique properties—different from the metals they are created from to achieve exceptional properties like hardness, appearance, durability, and other attributes. Products and parts made from alloys that align with the alloy’s strengths are reliable and often are aesthetically and functionally superior.
Aluminum alloys are common materials of choice for many engineers. Aluminum alloy products and parts are
light, strong, resilient, ductile at low temperatures, corrosion-resistant, non-toxic, heat-conducting, reflective, electrically conducting, non-magnetic, non-sparking, and non-combustible. Some of the more common aluminum alloys that Ermak pours are A356 / 356 / 333 / C355 / 319 / 535 / and 713.
Bronze alloys are also known as workhorse metals used in a range of industries, from aerospace to marine. Bronze is one of the most versatile and corrosion-resistant alloys known to man. Ermak’s network of foundries pours bronze alloys C862 / C863 / C865 / C876 / C87610 / C89833 / C903 / C905 / C958
The distinction between alloying and impurity is not always clear; for example, when silicon is added to aluminum, it may be considered an impurity or a valuable component depending on the application. Silicon increases strength while decreasing corrosion resistance.